Analgesic Anti-inflammatory Patches
RAKOOL Inc., Japan.
Intenurse- Indomethacin patches for Topical pain relief - Pharmacological details
Characteristics & Warnings about usage
Tests to prove efficacy & safety
1.
Contents:
One patch weighs 14.0g contains 70mg of Indomethacin. Edetate Sodium
as additive.
2.
Physical
Characters:
Mild yellowish White medication applied with a combination of water on a
plain sheet of stretchable cloth. Mild odour. Size 10CM X 14 CM Mfg Code:
TOKO 301.
3.
Pharmacological
characters:
Indomethacin (Generic name)
1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic
acid. C19H16ClNO4.
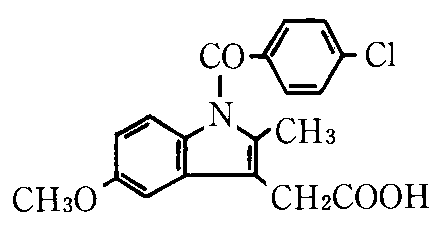
Mild
yellowish white colored granular powder with no odour. Insoluble in water,
and slightly soluble in methanol and ethanol. Soluble in NaOH. Color changes with exposure
to light. Melting point: 155-162 degree Celsius.
4.
Indications:
Arthritis deformans, Perichondritic arthritis, Frozen shoulder, Tendonitis, Bursitis,
Tennis elbow, Muscle ache, Sprains, Inflammation due to blunt injury,
Chronic arthritic pain and inflammation.
5.
Dosage:
once or twice on the affected area
1. Caragenin induced inflammation test
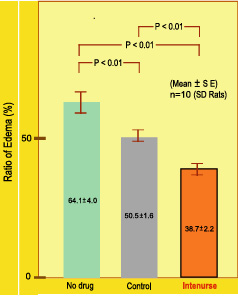
Caragenin was used in the hind limbs of rats to induce inflammation and the efficacy of Intenurse in controlling the inflammatory edema was studied. When the edema was 64%without treatment, the same was 50.5% with the control and 38.7% with intenurse.
6-1:
General Warning:
a.
This drug gives symptomatic relief from inflammation and pain
due to causes like injury etc but it doesnft treat the underlying cause
of such symptoms.
b.
On areas with infection, this has to be used in addition to
treating the infection.
c.
Chronic disorders may warrant additional treatment apart from
this.
d.
When using for long periods, beware of direct and indirect
adverse reactions.
6-2:
Contra indications:
a.
Donft@use
in patients who have past history of allergy to any form of Indomethacin
or similar group of drugs.
b.
Donft use in patients who have allergy or symptoms of
Asthma followed by usage of Aspirin or anti-histamines.
6-3:
Extra care to be applied while using in patients with Bronchial asthma as
this may provoke the existing Asthmatic problem.
6-4:
Adverse reactions: 0.1-5%: Mild redness, irritation. In less than 0.1%:
mild irritation and mild redness. When such symptoms are aggravated
consult the physician and stop the usage.
6-5:
Warning to pregnant women: Please inform your physician about pregnancy as
the safety of this medication during pregnancy has not been established.
In children and infants, safety of this medication has not been
established.
7.
Donft use this product on mucous membrane, ulcer or open wounds and
Eczema. When necessary fix the patch with surgical adhesive tape.
2. Effect on capillary permeability
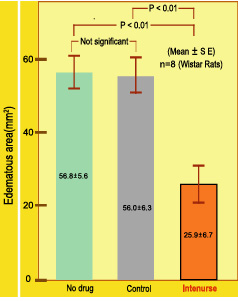
Capillary permeability increases with inflammatory response. In a laboratory test on Rats, when induced with a dye, without treatment, the area of edema due to capillary permeability was 56.8sq.mm, with control 56.0 sq.mm and with Intenurse it was 25.9 sq.mm.
8. This product is to be sold to or on order by a physician.
9. Store in a dry cool place away from direct sunlight. Keep the zipper tightly closed upon opening.
10. Use this before the date of expiry (printed on the outer box)
11. Packing: 5 Pcs/ pack: 100 packs/ Carton box.
@
|
3. Results of Clinical Skin irritation test:
Subjects: n=42 (Male 19;Female 23) age 20~ 58 1:
swelling with vesicle with redness; 2. swelling with redness; 3.
redness; 4: mild redness; 5. No reaction. ;
Patch test method: 10X10mm size patches were pasted using a 25 mm
surgical adhesive tape on the forearm of the subjects.
|
For enquiries contact us at +91-44-23720805 or by email: nbs@nichimail.jp
